Multicomponent Synthesis of Cyclic Depsipeptide Mimics by Ugi Reaction Including Cyclic Hemiacetals Derived from Asymmetric Organocatalysis
de la Torre,A. F.; Rivera, D. G.; Concepción, O.; Echemendia, R.; Correa, A. G. ; Paixão, M. W. J. Org. Chem., 2016, 81, 803–809. DOI: 10.1021/acs.joc.5b02158
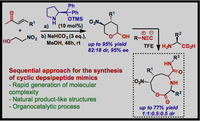
The synthesis of novel cyclic depsipeptide mimics by means of an organocatalytic conjugate addition, leading to chiral cyclic hemiacetals, followed by a multicomponent reaction with α-amino acids and isocyanides, is described. The initial organocatalytic step is employed for the asymmetric derivatization of α,β-unsaturated aldehydes to 4,5-disubstituted 2-hydroxytetrahydropyrans, which are next used as chiral bifunctional substrates on the Ugi five-center three-component reaction, giving rise to nine-membered-ring lactones.